MICROSCOPES and LABORATORIES
 |
 |
 |
| MAKING A THIN SECTION | LIGHT MICROSCOPES | SCANNING ELECTRON MICROSCOPE |
 |
 |
 |
| TRANSMISSION ELECTRON MICROSCOPE |
ELECTRON MICROPROBE: MINERAL ANALYSIS | MASS SPECTROMETERS: DATING ROCKS |
HOW BIG IS SMALL?
Our microscopes look at tiny pieces of rocks. The best way of measuring small distances is with the metric scale. The metric scale starts with a unit called a meter which is a little bit bigger than a yard.

Divide a meter into a hundred pieces and you have a centimeter (cm). There are about two and a half centimeters in an inch.
Divide a meter into a thousand pieces and you have a millimeter (mm). You can see millimeters easily on a ruler.
Divide a millimeter into a thousand pieces and you have a micrometer (mm). There are a billion micrometers in a meter.
Divide a micrometer into a thousand pieces and you have a nanometer (nm). There are a trillion nanometers in a meter.
The metric system also measures big distances. A kilometer (km) is a thousand meters. A kilometer is a little more than half a mile.
[pictured above] A millimeter is the smallest measure we can see on a ruler. One thousand millimeters make a meter. A meter is a little bit bigger than a yard.ATOMS, ELEMENTS, MINERALS AND ROCKS
Atoms are made up of a nucleus that contains protons and neutrons, and electrons that circle around the nucleus.
A chemical element is material made of only one type of atom. All the atoms of any one chemical element have the same number of protons in their nucleus. A helium balloon is filled with helium atoms that all have three protons in the nucleus. A sheet of aluminum foil is made of aluminum atoms that all have thirteen protons in the nucleus.
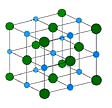
A mineral is a crystalline substance. A mineral can be made of only one element. An example is diamond which is made of carbon. Or, a mineral can be made of more than one element. An example is salt which is made of sodium and chlorine. Minerals that make up rocks usually contain silicon, oxygen and other elements like magnesium, aluminum, iron and calcium. Each mineral has its own chemical composition, and its own arrangement of atoms.
Rocks are made up of many small mineral grains that are put together in different ways depending on how the rock formed.
[pictured above]This model shows you how the atoms in a mineral are organized. It is a model of salt, the mineral we eat every day. Salt contains the elements sodium and chlorine. In the model, the blue circles are sodium atoms and the green circles are chlorine atoms. The atoms are arranged in a very organized pattern that is repeated over and over, throughout a single mineral grain.A THIN SECTION

Scientists look at thin slices of rocks under a microscope to learn more about what the rocks are made of.
A thin section is a highly polished slice of a rock that is thin enough for light to pass through. A thin section is 30 micrometers thick (that's three hundredths of a millimeter). To make a thin section, we first cut a slice of the rock that is about a millimeter thick, using a rock saw. Then we glue the slice to a glass slide, grind it down until it is the right thickness, and finally polish it until it is as smooth and shiny as a mirror.
LIGHT MICROSCOPE

A light microscope, or optical microscope, is used to study a thin section of a rock. The section is so thin that light passes through the rock.
Light in an optical microscope passes through two polarizing plates that are like the lenses in polarized sunglasses. Different minerals can be identified because polarized light passes through them in different ways. We can easily look at grains that are a few millimeters down to about 10 micrometers across.
Thin sections viewed in plane polarized light (one polarizing plate) are usually not very brightly colored. Different minerals are slightly different colors and often have different shaped grains. Minerals like metals, sulfides and oxides are opaque, which means that light does not pass through them. We see these as black grains in plane polarized light.
 |
 |
With crossed polars (two polarizing plates at right angles to one another), many minerals show bright colors and the thin section looks like a kaleidoscope. The minerals olivine and pyroxene are usually green, pink, yellow and blue, and feldspars and quartz are usually grey. In cross-polarized light, we can see twinning in minerals, differences in chemical composition and many other characteristics that help us understand how the rock formed.
[top picture above] Light microscope (optical microscope) at the University of New Mexico. This microscope uses transmitted light that passes through the sample, as well as reflected light that is reflected off the surface of the sample. The sample, usually a thin section of a rock, sits on the round black platform. [center picture above] Thin section photograph of the SAH99555 angrite (achondrite), taken in plane polarized light. The long, thin, white crystals are plagioclase feldspar and the pink crystals are pyroxene. The image is 2 mm across. [bottom picture above] Thin section photograph of the SAH99555 angrite (achondrite), taken in cross-polarized light. The long, thin, white and grey crystals are plagioclase feldspar and the brightly colored crystals are pyroxene. The image is 2 mm across.SCANNING ELECTRON MICROSCOPE
In a scanning electron microscope (SEM), pictures are made with electrons instead of light.
An SEM can be used to look at a 3-dimensional piece of rock a few centimeters across, or a polished thin section. The sample is placed under a beam of electrons. Images are always black-and-white. With an SEM we can take pictures of objects that are about a micrometer across.
 |
 |
 |
An SEM can make several different kinds of images. A secondary electron image (SE image) looks at electrons that are reflected off the sample. The image looks just like a picture you would take with your camera. A back-scattered electron image (BSE image) makes a picture in which the grey level is related to the atomic number (number of protons) of each grain. A grain of iron appears very white because iron has a high atomic number, and a grain of quartz (silicon and oxygen) appears dark grey because it has a low atomic number.
[above left]Scanning electron microscope (SEM) laboratory at the University of New Mexico. This instrument is used to take photographs of rocks using electrons that scatter off the surface of the sample. The white cylinder is the column for the electron beam. [above center]SEM image of a chondrule from the Mokoia (CV3) carbonaceous chondrite. This is a secondary electron image. The chondrule is like a ball, made mostly of pyroxene crystals that poke out of the surface. The image is 1.3 mm across. [above right]SEM image of the Bishunpur (LL3.1) ordinary chondrite. Round objects are chondrules. White grains are iron-nickel metal and iron sulfide. Grey grains are silicate minerals, mostly olivine and pyroxene. The different shades of grey show different chemical compositions. The white line at the top of the image is a 1 mm scale bar.TRANSMISSION ELECTRON MICROSCOPE

In a transmission electron microscope (TEM), pictures are made with electrons that pass straight through the sample.
For electrons to pass through a mineral grain, the grain must be about a tenth of a micrometer thick. One way to prepare a sample for a TEM is to take a thin section and make a hole in it with high-energy ions. Around the edge of the hole the grain is thin enough for TEM imaging. The sample is placed under a beam of electrons with very high energies. We use the TEM to look at objects that are a few micrometers down to a few nanometers in size.
[above left]Transmission electron microscope laboratory at the University of New Mexico. This instrument is used to take photographs using electrons that pass right through the sample. The tall white cylinder is the column for the electron beam. [left]TEM image of tiny grains of magnetite in the martian meteorite, ALH84001. Each grain is about 10 nanometers across.MINERAL ANALYSIS - ELECTRON MICROPROBE

An analysis of a mineral tells us what chemical elements it is made of. This helps us understand how the mineral grain formed.
We can analyze a mineral using an electron microprobe. This machine is like a scanning electron microscope (see above) but it has extra attachments called spectrometers that are used for analyses.
An electron microprobe can be used to determine which elements are present in a mineral, and how much of each element there is. An electron microprobe is like a scanning electron microscope: a sample is put underneath a beam of electrons. Where the electrons hit the sample, X-rays are given off. Each element gives off X-rays of different energies. We collect the X-rays and use them to determine the composition of the mineral. Each analysis tells us the composition in a spot that is about 1 micrometer across.
 [top picture above]Electron microprobe at the University
of New Mexico. This instrument is used to take pictures
like a scanning electron microscope. It is also used to
measure the chemical composition of mineral grains. The
tall white cylinder is the column for the electron beam.
It is surrounded by spectrometers that are used to measure
the X-rays that come off the sample when it is hit by the
electron beam.
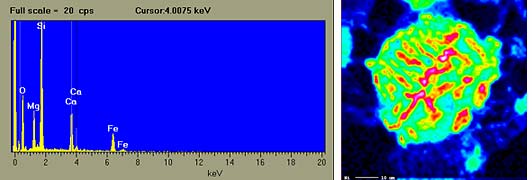
[above left]X-ray spectrum for pyroxene. X-rays are produced when atoms
are hit by an electron beam. Each element in the pyroxene
grain emits X-rays with a distinct energy. The pyroxene
grain that was analyzed here contains oxygen (O), magnesium
(Mg), silicon (Si), calcium (Ca) and iron (Fe).
[above right]X-ray image of a metal grain in the PAT 91546 (CH3) carbonaceous
chondrite. The image is made with X-rays that have been
emitted from nickel atoms. The green circle is the grain
of metal, which is a mixture of iron and nickel. Red patches
show where there is a lot of nickel. The white line in the
bottom left corner of the image is 10 micrometers long.
[top picture above]Electron microprobe at the University
of New Mexico. This instrument is used to take pictures
like a scanning electron microscope. It is also used to
measure the chemical composition of mineral grains. The
tall white cylinder is the column for the electron beam.
It is surrounded by spectrometers that are used to measure
the X-rays that come off the sample when it is hit by the
electron beam.
[above left]X-ray spectrum for pyroxene. X-rays are produced when atoms
are hit by an electron beam. Each element in the pyroxene
grain emits X-rays with a distinct energy. The pyroxene
grain that was analyzed here contains oxygen (O), magnesium
(Mg), silicon (Si), calcium (Ca) and iron (Fe).
[above right]X-ray image of a metal grain in the PAT 91546 (CH3) carbonaceous
chondrite. The image is made with X-rays that have been
emitted from nickel atoms. The green circle is the grain
of metal, which is a mixture of iron and nickel. Red patches
show where there is a lot of nickel. The white line in the
bottom left corner of the image is 10 micrometers long.
DATING ROCKS - MASS SPECTROMETERS

We can find out the age of a rock using natural radioactivity that can be used like a clock.
All rocks contain very small amounts of elements that are naturally radioactive. Some of the atoms of the radioactive elements are changing into atoms of other elements. For example, atoms of the element rubidium change into atoms of strontium. This happens very slowly and can take billions of years. If we measure how much strontium is in the rock, we can tell how old the rock is. We can make these measurements using a mass spectrometer.
Atoms of the same element that have different numbers of neutrons in their nucleus are called isotopes. For example, a hydrogen atom can have one, two or three neutrons in its nucleus: we call these isotopes hydrogen, deuterium and tritium. Not all isotopes of an element are radioactive. The rubidium-strontium clock used to date rocks looks only at the decay of the rubidium-87 isotope (87Rb) to the strontium-87 isotope (87Sr). The mass spectrometer measures the concentrations of elements that are only about one billionth of the total mass of the rock. The rock must be dissolved in acid in order to make these measurements.
[pictured above]Mass spectrometer at the University of New Mexico. This instrument is used to date rocks. The rock is dissolved in acid and each chemical element is separated. The amounts of each isotope are measured in the mass spectrometer, and the isotopes are used to determine the age of the rock.HOME ||| PLANNING YOUR VISIT ||| INSTITUTE OF METEORITICS ||| UNM
All rights reserved. No reproduction without written permission. Design and photography by Stanegate Studios and Karl Jay Designs